Supporting remote cardiac rehabilitation Remohab
Remohab (Osaka City, President Tatsunori Taniguchi) has received approval for manufacturing and sales (pharmaceutical approval) for the medical device program “Remohab CRU” that supports remote cardiac rehabilitation.
This is the first pharmaceutical approval in Japan for a medical device program that remotely supports rehabilitation.
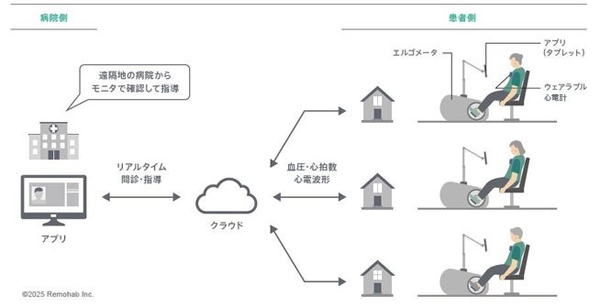
This program supports remote cardiac rehabilitation for one or multiple patients at the same time under the supervision of a medical professional in an online environment. By combining the medical device ergometer “Remohab cycle+” (generic name: non-active extension/flexion rotation exercise device) developed by the company with the wearable electrocardiogram “Remohab rhythm+” (telemetry-type electrocardiogram transmitter), patients can safely perform cardiac rehabilitation at home under the online supervision of a medical professional without having to visit a medical institution.
Heart disease is the second leading cause of death in Japan. Cardiac rehabilitation is known as an important treatment for heart disease, along with drug therapy.
Traditionally, cardiac rehabilitation immediately after discharge was conducted while visiting a medical institution, but the implementation rate is extremely low, with only 7.1% of hospitalized patients with heart failure receiving the treatment. The reason for this is that cardiac rehabilitation is standardly conducted three times a week, which requires frequent visits to a medical institution, creating an obstacle.
※Translating Japanese articles into English with AI