Shortening the development period for therapeutic drugs NTT Communications
NTT Communications (NTT Com) and I’rom Group will collaborate with the aim of shortening the development period for therapeutic drugs.
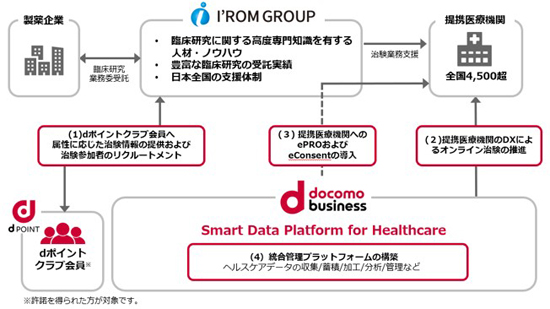
The collaboration will combine the clinical research support system that I’rom Group has provided to over 4,500 affiliated medical institutions nationwide with NTT Com’s ICT solutions to shorten clinical trial periods, improve efficiency, and improve quality.
As a first step, They will provide clinical trial information and recruit trial participants to NTT Docomo’s 7 million d Point Club members, the Premier Panel.
By providing information on new treatments and new drugs and introducing medical institutions that can conduct clinical trials within their living area, they create an environment where members can feel more familiar with clinical trials and actively participate, and encourage them to participate in clinical trials. They aim to secure personnel as early as possible.
In Japan, sufficient treatments have not been established for cancer, immune disorders, neurodegenerative diseases, diabetes, mental disorders, etc., and there is a need for the development of new drugs.
On the other hand, it takes longer to approve and distribute therapeutic drugs than in other countries, and it is said that approximately 72% of new drugs distributed in the United States and the European Union are not approved domestically, resulting in drug loss.
This problem is due to the difficulty in securing trial participants, lack of resources at medical institutions conducting trials, and underdeveloped infrastructure.
The collaboration will promote the development and dissemination of new drugs by fundamentally improving the clinical trial implementation system, shortening the trial period by securing trial participants, and improving efficiency and quality by utilizing digital tools.